
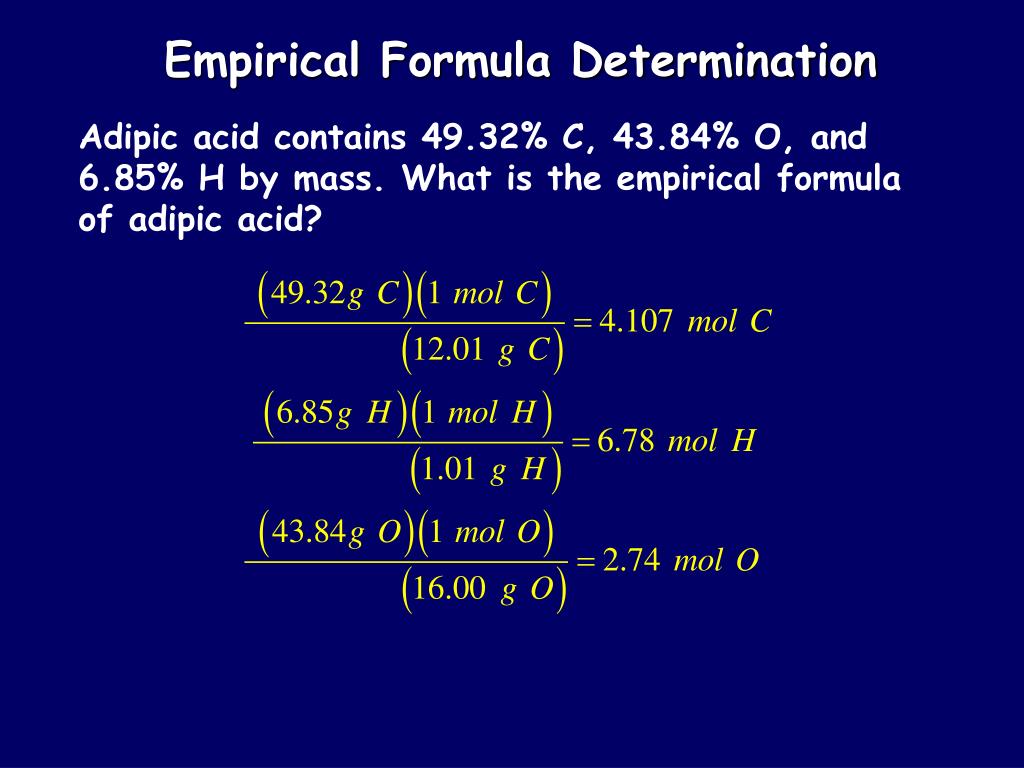
modern 1.008 14.007 15.9992) Finally, the chemical analyses to which Dalton had access put the O:N mass ratio in NO between 1.15:1 and 1.36:1. Summary of atomic weights of H, N, and O used in exercise 1. This is the result that an accurate analysis would obtain. Use these atomic weights to predict the O:N mass ratio in NO.Ĭ) Use the atomic weights of nitrogen and oxygen from a modern table to predict the O:N mass ratio in NO. Since Dalton assumed that the formulas of water and ammonia were HO and NH respectively, he would have supposed that the atomic weights of oxygen and nitrogen were 7.94 and 4.63, respectively. By weight, the ratio of oxygen to hydrogen in water is 7.94:1 and the ratio of nitrogen to hydrogen in ammonia is 4.63:1.
Dalton decided to use hydrogen as the unit for his system of atomic masses. Use these atomic weights to predict the O:N mass ratio in NO.ī) Now suppose Dalton had had accurate analyses of water and ammonia available in setting up his system of atomic weights. Consider a testable prediction based on the following sets of atomic weights: predict the mass ratio of oxygen to nitrogen in " nitrous gas" if the formula is NO.ġa) In the atomic weight scale Dalton began to construct, the atomic weights assigned to nitrogen (" azote") and oxygen were 5 and 7 respectively. The theory was not correct in every respect however, it was a pivotal advance in chemistry because it had consequences that could be tested by experiment. Dalton atomic weights Dalton atomic weightsJohn Dalton is remembered today for a remarkably fruitful atomic theory.


 0 kommentar(er)
0 kommentar(er)
